Market Access & reimbursement for digital health applications
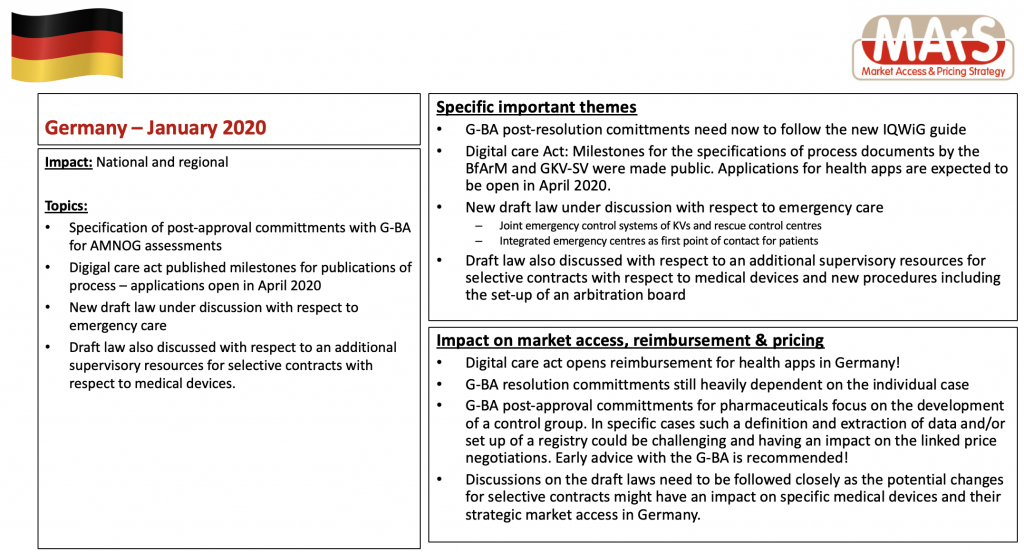
In Germany, a new market access and reimbursement process for digital health applications (DiGAs) was introduced in early 2020. In our approx. 45-minute webinar, you will learn what this new process looks like and which similar processes may exist in a European comparison. The French expert Guy Eiferman from the Market Access consulting agency Nextep will speak about the French process. The webinar will conclude with a panel discussion on the main differences and similarities between France and Germany as driving forces for digital innovation in Europe.