Access to German hospitals – pathways to follow
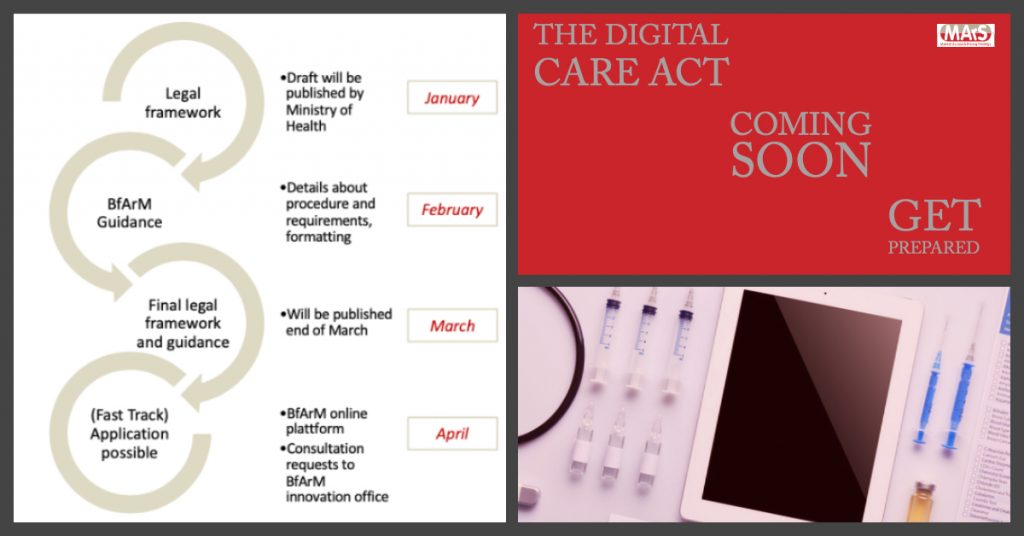
The pathway to German hospitals for medical devices, digital health applications and drugs can be straight forward in Germany. However, in the meantime there are various pathways available including the famous “NUB” and the newly introduced Hospital Future Act as well as experimental coverage and innovation fund applications. Additionally, investment goods need to differentiate between hospital ownerships.
The presenters, both long-lasting members of the Inpatient committee at the German Health Economic Association, will discuss the various pathways and applicability as well as the correlation to the annual budget negotiations of hospitals and will show the various aspects to consider when planning a launch into the German hospital setting.